lv wcd | wcd vs vf lv wcd The wearable cardioverter-defibrillator (WCD) is a device designed for patients . Find many great new & used options and get the best deals for Pokemon Gengar Lv.X Advent of Arceus 1st Edition Japanese Holo #043 PSA 10 at the best online prices at eBay! Free shipping for many products!
0 · what is a wcd device
1 · what is a wcd
2 · wcd vs vf
3 · wcd indications
4 · wcd icd
5 · wcd for sale
6 · wcd defibrillator
Browse Las Vegas area obituaries on Legacy.com. Find service information, send flowers, and leave memories and thoughts in the Guestbook for your loved one.
Reg: LV-WCD photos; Aircraft: Cessna 172RG Cutlass RG; Serial #: 17RG0832; . The wearable cardioverter-defibrillator (WCD) is a device designed for patients . In cases where ICD implantation must be deferred, a wearable cardioverter . Clinical data were captured for arrhythmic events, ICD implantation, and .
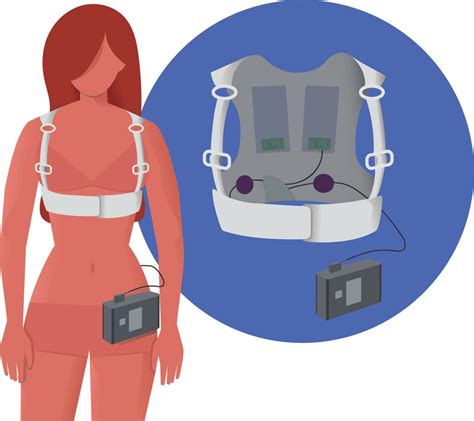
Reg: LV-WCD photos; Aircraft: Cessna 172RG Cutlass RG; Serial #: 17RG0832; Photo date: 2011-03-13; Uploaded: 2011-03-27 The wearable cardioverter-defibrillator (WCD) is a device designed for patients at risk of SCD who are not immediate candidates for ICD therapy. By providing automatic therapy, the WCD does not depend on a second person to defibrillate, as required with a manual or automated external defibrillator (AED). In cases where ICD implantation must be deferred, a wearable cardioverter-defibrillator (WCD) offers an alternative approach for the prevention of SCD.
Clinical data were captured for arrhythmic events, ICD implantation, and improvement in left ventricular ejection fraction (LVEF). The median age was 62 years; the median LVEF was 25%. The median WCD wear time .The WCD has been increasingly used for primary prevention of SCD during the high-risk gap periods early after MI, coronary revascularization with CABG or PCI, or new diagnosis of heart failure, when its use is as a protective bridge to ICD or LV improvement. The wearable cardioverter-defibrillator (WCD) was first approved for clinical use in 2002, and is routinely used in select populations at high risk for sudden cardiac death. WCDs are frequently considered as a bridge to definitive therapy or in circumstances where insertion of conventional implantable cardioverter-defibrillators (ICD) is .
what is a wcd device
The wearable cardioverter-defibrillator (WCD) was approved by the US Food and Drug Administration in 2001. Since then, use of the WCD has grown steadily, and the device is now also available in Australia, Europe, Israel, Japan, and Singapore. The use of the wearable cardioverter-defibrillator is included in the new 2015 ESC guidelines for the management of ventricular arrhythmias and prevention of sudden cardiac death. The present review provides insight into the current technology and an overview of this approach. The wearable cardioverter-defibrillator (WCD) is an alternative antiarrhythmic device that provides continuous cardiac monitoring and defibrillation capabilities through a noninvasive, electrode-based system.
The most common clinical indication for WCD-prescription (63%) was a new diagnosis of severely impaired LV function (LVEF ≤35%). The median wear time of the WCD was 54 days with a daily use of 23 h. Reg: LV-WCD photos; Aircraft: Cessna 172RG Cutlass RG; Serial #: 17RG0832; Photo date: 2011-03-13; Uploaded: 2011-03-27
women's long prada coat
The wearable cardioverter-defibrillator (WCD) is a device designed for patients at risk of SCD who are not immediate candidates for ICD therapy. By providing automatic therapy, the WCD does not depend on a second person to defibrillate, as required with a manual or automated external defibrillator (AED). In cases where ICD implantation must be deferred, a wearable cardioverter-defibrillator (WCD) offers an alternative approach for the prevention of SCD. Clinical data were captured for arrhythmic events, ICD implantation, and improvement in left ventricular ejection fraction (LVEF). The median age was 62 years; the median LVEF was 25%. The median WCD wear time .The WCD has been increasingly used for primary prevention of SCD during the high-risk gap periods early after MI, coronary revascularization with CABG or PCI, or new diagnosis of heart failure, when its use is as a protective bridge to ICD or LV improvement.
The wearable cardioverter-defibrillator (WCD) was first approved for clinical use in 2002, and is routinely used in select populations at high risk for sudden cardiac death. WCDs are frequently considered as a bridge to definitive therapy or in circumstances where insertion of conventional implantable cardioverter-defibrillators (ICD) is . The wearable cardioverter-defibrillator (WCD) was approved by the US Food and Drug Administration in 2001. Since then, use of the WCD has grown steadily, and the device is now also available in Australia, Europe, Israel, Japan, and Singapore. The use of the wearable cardioverter-defibrillator is included in the new 2015 ESC guidelines for the management of ventricular arrhythmias and prevention of sudden cardiac death. The present review provides insight into the current technology and an overview of this approach.
The wearable cardioverter-defibrillator (WCD) is an alternative antiarrhythmic device that provides continuous cardiac monitoring and defibrillation capabilities through a noninvasive, electrode-based system.
what is a wcd
prada bags sale women
prada cloudbust sneakers women size 11
5 Underrated Louis Vuitton Bags That Are Better Than the Icons. No Neverfulls or Twist Bags in sight. Photo Courtesy of Getty Images. BY Jordan Goldberg And Kate Bernard. Last Updated. Jan 15, 2024. When you think of Louis Vuitton handbags, what comes to mind?
lv wcd|wcd vs vf

























